Introduction
Foot and mouth disease (FMD)also called Aphthous fever is a highly contagious disease that affects cloven-footed animals( cattle, buffalo, goat, sheep, pig, camel, and cloven-footed wildlife species) and is named vesicular lesions mostly found in and around the mouth and on foot and in lactating females mostly of cattle lesions may found on teats.
Etiology of FMD
The foot and mouth disease virus (FMDV) is a nonenveloped RNA virus of the viral family Picornaviridae and genus Aphthovirus that causes FMD.
Serotypes of FMDV
Foot and mouth disease virus (FMDV) has 7 major serotypes (A, O, C, Asia 1, SAT 1, SAT 2, SAT 3).
Epidemiology of FMD
Foot and mouth disease affects all cloven-footed animals, and there have been three major outbreaks of foot and mouth disease (FMD). The first outbreaks of FMD occurred in Great Britain in 2001,
another outbreak occurred in the Republic of Korea in 2010/2011, and a third major outbreak of foot and mouth disease (FMD) occurred in Japan in 2010. FMD outbreaks in North America, the last outbreak in the United States occurred in 1929, Canada in 1951-1952, and in Mexico in 1946-1954.
- Serotypes of foot and mouth disease(FMDV) in Africa are 6 out of a total of 7.
SAT 1, SAT 2, SAT 3, A, O, and C. - Serotypes of foot and mouth disease(FMDV) in Asia are 4
A, O, C, Asia 1. - Serotypes of foot and mouth disease(FMDV) in South America are 3
A, O, C.
Prevalence of Foot and Mouth Disease
There is no specific data about FMD prevalence in different countries.
Usually, serotypes A and O are responsible for most outbreaks. Outbreaks with serotype C are uncommon.
Morbidity and Case Fatality in FMD
Morbidity in FMD outbreaks in susceptible animals may reach 100%
But case fatality is very low in older animals, about 2%, and a bit high in young animals, about 20%.In 1997, an outbreak in Taiwan caused Case fatality in pigs to be 18% but reached 100%.
Transmission of FMD
Transmission of FMD occurs from one animal to another by aerosols and ingestion. In transmission, direct contact is very important in FMD spread.
Spread of FMD from pigs to cattle by the movement of people, slaughterhouse waste, or animals. Cattle are more susceptible to airborne transmission. FMDV can spread about 250km by using water as a medium and 100km by land.
Serotype A takes a shorter time of exposure for contact transmission than serotypes O and C.
FMDV is shed in saliva, semen, and milk after viremia before showing clinical signs of pressure of the mouth, teeth, and foot lesions.
Animals during convalescence or those that receive vaccines are carriers. The virus of FMDV passes UN changed through the git of birds and can be a source of long-distance virus transmission by acting as carriers.
Risk factors for FMD
Host-specific
Cattle and pigs are more susceptible to FMD. Goats are infrequent carriers of FMDV, but sheep are not at all.
Equines are not suspectable for FMD.
In camelids, dromedaries are not susceptible to FMD, but Bactrian camels are susceptible to FMD.
African buffalo are a source of the SAT virus.
Asian buffalo are also susceptible to FMD and can spread to one another.
Environmental factors
FMD virus can persist for 1 year in infected premises and 10- 12 weeks on clothes and feed, and also in semen for more than 60 days frozen to -97C°(110°F).
Viruses can be destroyed by direct sunlight, but low temperature enables their presence on feed and other materials.
The virus can be destroyed in minutes using disinfectants such as sodium hydroxide or formalin and sodium carbonate.
Pathogenesis of FMD
Usually involves three phases
1) Previremic phase
It involves infection and replication at replication sites and lasts for 3 weeks.
The primary site of replication in Cattle is the nasopharyngeal region.
FMDV affects innate immunity by blocking interferon response and also influences natural killer cells to recognize and eliminate FMDV.
2) Viremic form
It is characterized by the presence of the virus in blood and is disseminated to epidermal sites of the mouth, feet, teats, and snouts of pigs and lasts for 1 to 21 days.
Viruses are shed in this phase in urine, milk, semen, saliva, inhaled air, and feces for 2 weeks.
3) Postviremic
It is characterized by the healing of lesions
of FMD in the mouth, feet, snouts of pigs, and teats of lactating cows.
Oral lesions heal more rapidly than feet and teats.
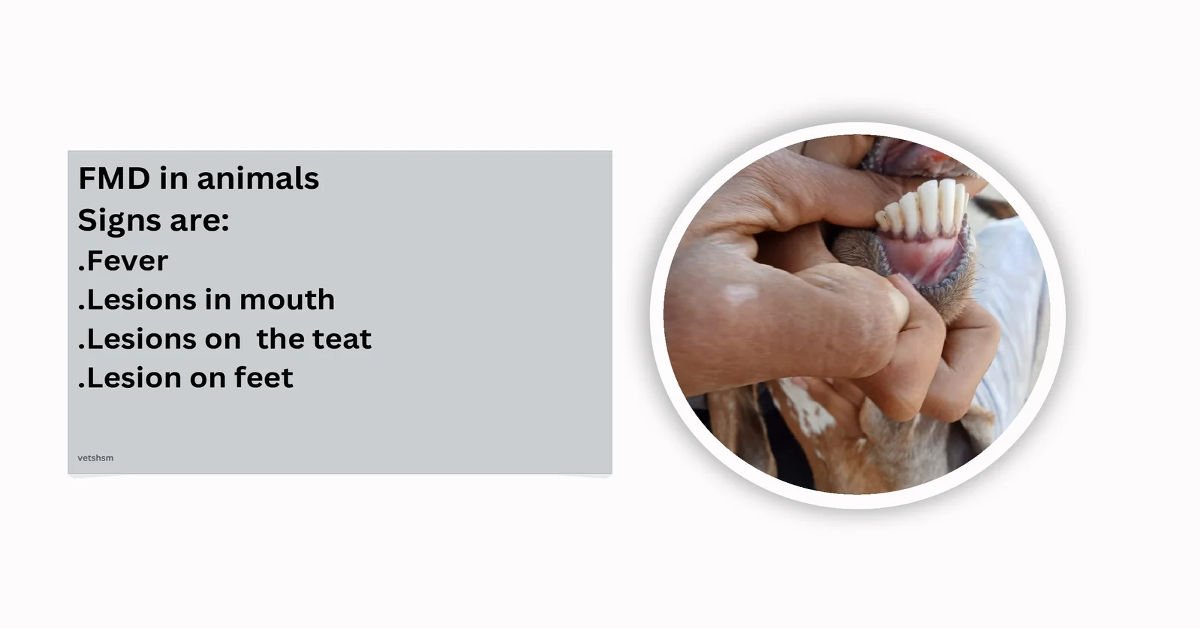
Clinical findings
Animals affected with foot and mouth disease show the following clinical findings:
Fever 104 – 106, C°
A fall in milk yield
. Anorexia
.Painful stomatitis
. Salvation (Sting-like rope )
. Vesicles on the buccal mucosa, dental pad tongue, feet, coronet, snouts, and teats.
. Lameness
. Recumbent
. Mastitis
.Off feed
. Necrotic myocarditis in young animals
. Dysentery
. Diarrhea
. Enteritis
Sequelae of FMD
. Decrease milk production
. Hypertrichosis (hairy panter)
. Pituitary damage-related dyspnea syndrome
Heat intolerance
The sequelae of hairy panter is mostly present in European cattle breeds.
Differential diagnosis by
- Vesicular Stomatitis:
- Vesicular Exanthema:
- Swine Vesicular Disease
- Bovine Viral Diarrhoea (BVD
- Rinderpest
- Malignant Catarrhal Fever (MCF)
Treatment of FMD
No anti-viral treatments in food animals, but supportive and symptomatic
.Penicillin
. Use protective dressing to prevent secondary infection
.NSAIDS (Flunixin meglumine)
. Ethnoveterinary remedies like soda ash washing and applying honey and finger millet flour for 3 days.
. Porcine type 1 interferon
. Bovine type 3 interferon
Control:
Vaccination in endemic areas with killed vaccine R-1.
. By maintaining biosecurity
. Restricted movement and trade of infected animals
Vaccination of FMD
Use the killed trivalent vaccine (A, O, C) in infected animals. A single dose provides immunity for 6 or 7 months.
Cattle must be vaccinated every 6 months. Calves from unvaccinated dams are vaccinated at age 4 months and revaccinated at 8 months. Calves from vaccinated dams are vaccinated at age 6 months and revaccinated at 10 months of age. Goats and sheep aren’t included in the vaccination program unless outbreaks occur.
Zoonotic status of FMD
Humans are considered to be susceptible to viruses and may contain lesions on the hands and mouth, but cases are few except those that work with the carcasses of animals and laboratory personnel. Moreover, humans and their clothes are a source of virus transmission.
Innovative Diagnostic Approaches
Swift and precise diagnosis is essential for containing FMD outbreaks. Recent advancements have enhanced molecular diagnostic tools, particularly real-time reverse transcription polymerase chain reaction (RT-PCR). This assay’s high sensitivity enables identification of the virus in samples with low viral loads, such as those from asymptomatic carriers, which can silently spread FMDV for months. Additionally, enzyme-linked immunosorbent assays (ELISAs) have been refined to support the differentiation of infected from vaccinated animals (DIVA), a critical tool for maintaining trade in FMD-free zones.
Next-Generation Vaccine Development
The genetic diversity of FMDV, with seven serotypes and multiple subtypes, poses significant challenges for vaccination. Conventional inactivated vaccines are effective but require serotype-specific formulations and cold-chain logistics, limiting accessibility in low-resource regions. Recent research has focused on developing versatile, stable vaccines. A 2023 study in a leading veterinary science journal introduced a recombinant adenoviral vaccine encoding FMDV capsid proteins for serotype A, eliciting strong immunity in goats within six days. This vaccine’s thermostability eliminates the need for refrigeration, making it ideal for remote areas. Similarly, mRNA vaccine platforms, inspired by human vaccine technologies, are showing promise. A 2025 trial in the Journal of Veterinary Medicine reported that an mRNA vaccine for serotype O induced protective antibodies in cattle within five days, with potential for rapid adaptation to emerging subtypes. These innovations aim to provide broader protection and simplify deployment in endemic regions.
Epidemiological Modeling and Control Strategies
Understanding FMDV transmission dynamics is crucial for effective control. Recent studies have leveraged computational models to predict outbreak patterns and optimize response strategies. A 2024 study in Preventive Veterinary Medicine used machine learning to analyze FMD spread in livestock markets, identifying high-risk trade routes in Africa. These models inform targeted vaccination campaigns, reducing outbreak scale by up to 40%. Additionally, research into carrier animals has clarified their role in silent transmission. This insight supports culling or quarantine strategies in endemic areas. Furthermore, global surveillance networks, strengthened by 2025 initiatives from the World Organisation for Animal Health, integrate real-time data sharing to track FMDV strains, enabling rapid response to cross-border outbreaks.
Public Health and Economic Implications
While FMD is not zoonotic, its economic impact indirectly affects public health by disrupting food security and livelihoods. Research emphasizes cost-effective control measures, such as regional vaccine banks and DIVA-compatible vaccines, to minimize trade disruptions. A 2024 economic analysis in Agricultural Economics estimated that improved diagnostics and vaccines could save $2 billion annually in affected regions.
Modern FMD research has made significant strides in diagnostics, vaccine technology, and epidemiological modeling, offering hope for better control of this devastating disease. Enhanced RT-PCR and DIVA ELISAs enable rapid detection, while adenoviral and mRNA vaccines promise broader, more accessible protection. These innovations, driven by 2023–2025 research, pave the way for reducing FMD’s global burden, safeguarding animal health, and economic stability.
FAQ’S
- What is FMD?
A highly contagious viral disease affecting cloven-hoofed animals, causing systemic illness with lesions in the mouth and feet, leading to significant economic and trade impacts. - Why is FMD important?
It disrupts livestock productivity, triggers trade embargoes, and requires costly control measures, making it a global concern for agriculture and food security. - How does FMD spread?
Through direct contact, aerosols, contaminated objects, and movement of infected animals or products highlight the need for strict biosecurity. - What are the key symptoms?
Fever, vesicles (blisters) in the mouth and on the feet, drooling, lameness, and a drop in milk production often resemble other vesicular diseases. - Can humans get FMD?
No, but humans can act as carriers, spreading the virus to animals through contaminated clothing, equipment, or vehicles.